Hormones are a hot-button topic. Broadly speaking, men want to be manly, women want to be feminine, and the steroid hormones—testosterone and estrogen—are the magic juice that make those things happen. And because sexuality and gender identity carry a lot of emotional weight, people tend to take notice of things that threaten their hormones—even if they might have a blasé attitude about other aspects of health (we all have that friend who rationalizes their smoking habit with the old refrain: “Eh, everything gives you cancer”). The existence of phyto-estrogens in soy has given rise to the term “soy boys”, used to deride men seen as unmasculine, while much of the legislation around what materials are food-contact-safe stems from research showing that components of certain plastics are estrogen-mimics that can impair fertility. It seems that, if there’s one case where even a vaccine skeptic will get behind the rallying cry of “believe the science”, it’s when their balls are on the line.
Even so, things aren’t looking good for the endocrine system of the average modern person. Hormone-linked cancers like prostate and breast cancer are major killers. Diseases like PCOS seem to be on the rise. And for years now, scientists have been sounding the alarm about widespread declines in sperm count, along with an apparent drop in average blood testosterone levels. But in spite of all this evidence that something is wrong, we’re still largely in the dark about what that might be.
That’s why it’s surprising to me that few people seem to be aware of the studies—now more than fifty years old—showing that the gut microbiome is a key node in hormone metabolism, and that antibiotics can seriously disrupt it.
That research dates back to the early 1970s, when scientists discovered that a standard prescription course of drugs like ampicillin results in major shifts to the body’s handling of estrogens and other steroid hormones. Decades before anyone had uttered the word “microbiome”, researchers had hit on some of the first hard evidence that gut bacteria are responsible for health far beyond the confines of the gut. Now, more than half a century later, we’re just beginning to reckon with the implications of their findings.
-sterols and Steroids
In a previous piece, we discussed enterohepatic recirculation, the process where your liver secretes cholesterol and bile acids into the GI tract, to help digest and dissolve1 nutrients. Those nutrients are absorbed in the small intestine, and the cholesterol goes with them—traveling through the bloodstream back to the liver, which will spit it back out into the intestines again. Each time a molecule of cholesterol makes that journey, gut bacteria have a chance to get their hands on it, transforming it into coprostanol—a chemical that can’t be re-absorbed by the intestines. As a result, you shit it out, which helps prevent cholesterol from clogging your arteries.
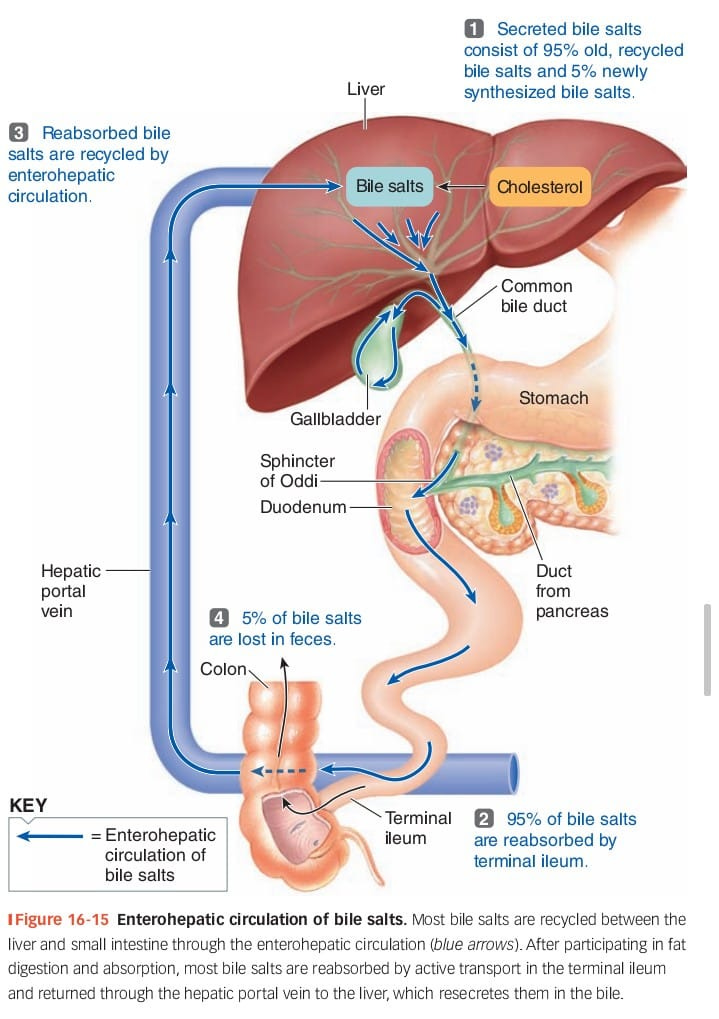
But bile acids and cholesterol aren’t the only kinds of compounds that make this trip. Estrogen and testosterone are described as steroid hormones because they’re produced in the body from cholesterol, and the liver handles them in a similar way: When a steroid passes through the liver, enzymes there can conjugate it—tagging it with a sugar or a sulfur molecule to deactivate it—and then dumping it into the gut to be pooped out.
Typically, though, that’s not the end for a hormone: certain species of gut bacteria possess glucuronidase enzymes that can rip that sugary “tag” off, and this reactivates the hormone and allows it to be reabsorbed in your intestines. The net effect is that—although every molecule of steroid hormone in the body gets sent into the GI tract for disposal at one point or another—the vast majority (about 90%) of hormones that actually make it out of the body do so via the urine. In short: where the microbiome’s complex chemistry helps us get rid of cholesterol, it does the opposite for hormones—helping them stick around longer than they otherwise would.
That’s why, when scientists in the late ‘60s and early ‘70s performed experiments to see how antibiotics impact the body’s hormone systems, the first thing they noticed was that they cause a temporary but alarming decrease in urinary levels of estrogen and a variety of related hormones. Before long, though, a Finnish physician named Herman Adlercreutz would discover that this sudden drop in urinary hormone levels was accompanied by a simultaneous rise in fecal levels of the same compounds, in sugar-tagged form. From this result, he and his colleagues deduced that antibiotics don’t interfere with the production of these hormones, but with their metabolism by gut bacteria: In a healthy body, the microbiome acts as a recycling service for hormones conjugated and secreted by the liver. When antibiotics decimate our bacteria, the recycling plant goes offline, and hormones instead end up going to waste as they’re excreted in stool.
Adlercreutz’s investigations found that, in the short-term, antibiotics don’t have a substantial impact on blood levels of hormones—just on whether they come out in stool or in urine. Even so, his reports contain hints that disturbances to the microbiome could pose a risk to hormonal health: the observed increase in fecal hormone levels was smaller than the decrease in urinary ones, leading him to speculate that bacteria in the colon were destroying the remainder.2 He also found that plasma levels of androstenedione, a precursor for testosterone production in the body, dropped significantly in male subjects by the end of a five-day course of antibiotics.
Adlercreutz’s work focused mostly on estrogens, but there’s growing evidence that testosterone is subject to the same processes—which might explain why certain antibiotics are known to tank testosterone levels in lab animals, accompanied by temporary infertility. Some of Adlercreutz’s contemporaries even reported measurable drops in blood testosterone in humans taking antibiotics. This effect wasn’t seen in Adlercreutz’s studies, potentially due to differences in dose, type of antibiotic, or the initial gut health of the subjects; his cohort in the study linked above consisted of five healthy men, and none got so much as a case of diarrhea from the antibiotic.
In most cases, the changes in hormone metabolism induced by antibiotics are temporary: you go off the antibiotic, your gut bacteria grow back, and everything is working normally again a few days later. That said, the microbiome is complex and hugely variable from person to person, so what’s true for most people may not be true for everyone. There’s a prime example of this in the research on antibiotics and cholesterol: for about one person in ten, a week of antibiotics is enough to semi-permanently annihilate the capacity for coprostanol production, putting them at higher risk of heart disease. If all you’ve got is a five-person study, it’s all too easy to miss a one-in-ten effect—which means there’s a real possibility that microbiome disruptions are at the root of some people’s hormonal woes.
Estrogen(s)
Why does the body even go through this weird rigamarole of conjugating hormones and sending them to the gut, only to have its work undone when the hormones are sent back into circulation by the microbiome? The answer may lie in the fact that deconjugation isn’t the only reaction that can happen while hormones are in the GI tract.
For background: The term “estrogen” doesn’t refer to a single chemical, but rather a closely-related group of compounds that govern the reproductive cycle in women. The three main ones are estrone, estradiol, and estriol. Estradiol is the most important, being 10 times as potent as estrone when it comes to activating estrogen receptors, and 100 times as potent as estriol (ordinarily a minor hormone, but one which plays a big role in pregnancy).
In 1980, Adlercreutz & co. reported their finding that certain Bacteroides species can transform estrone into the more powerful estradiol, while other microbes like Streptococcus can perform the reverse reaction—downgrading estradiol back into estrone. The body can carry out these same transformations on its own, but it seems likely that shuttling hormones into the gut is a way of outsourcing some of this work. This might be why some hormonal disorders like prostate cancer and endometriosis are linked to alterations in the microbiome.
But I think this kind of thing goes beyond questions of “health vs. disease”; I suspect it can explain some major aspects of people’s personalities. I recently found out that I have a bacterium in my gut which converts cortisol—the “stress” hormone that helps adrenaline do its thing—into a weird steroid derivative that prevents the synthesis of cortisol by your own cells. I’ve always joked that I have a pathological lack of anxiety,3 that I’m relaxed even when I shouldn’t be. It gets me into trouble sometimes (I’ve ended up way too high in a tree more times than I can count) but I also consider it one of my greatest strengths, and now I have to wonder if I owe it partly to this little self-limiting feedback loop in my gut that sets a cap on cortisol production.
Recent work4 has even shown that gut bacteria also have the capacity to produce neurosteroids—hormone derivatives that control the activity of neurotransmitters like GABA in the brain. One of these, allopregnanolone, was recently approved as a treatment for postpartum depression, but its short half-life means it has to be administered on an IV drip in the hospital, at a cost of something like $30,000 for a course of treatment. If gut bacteria produce this or similar compounds, they might provide a much cheaper and easier way to produce the same effect—as well as a potential explanation for why some people get PPD in the first place, if they’re lacking those bacteria.
The Takeaway
For starters, all of this is another powerful reason why everyone should be mindful of their microbes—and a potential leverage point in the fight against trends like antibiotic overuse. “Top-down” approaches like pressuring doctors to prescribe less don’t seem to work very well, in part thanks to the customer-service model of modern healthcare. If we want to curb the growing crisis of C. diff and other antibiotic-resistant superbugs, the easiest way to do it is to reduce the number of people walking into their doctor’s office demanding a Z-pack for their COVID. And the easiest way to do that is by educating people about the risks they’re taking—particularly when those pose a threat to near-universally treasured aspects of people’s identities, like sexuality and gender.
Even beyond the immediate public health implications, the microbiome is the greatest source of biological diversity among people—which is why I often say that if you’re looking for why one person is chronically ill and another is healthy, it’s the first logical place to check. But that logic doesn’t just apply to health vs. disease—it’s true of any biological variable. Knowing that hormone metabolism is under microbial control, it seems highly likely that a lot of the natural variation in things like testosterone levels is attributable to the presence or absence of certain bacteria.
Microbes are the future of health and medicine because they offer a natural, field-tested way of altering our biology—one that’s extremely simple compared to things like gene therapy. In the bacterial-utopian future, the probiotics aisle lets you pick genes a la carte: If you’re trying to age gracefully, a steroid-deconjugating probiotic might be a natural substitute for hormone replacement therapy. If you’re infertile and it turns out your estrone/estradiol ratio is out of whack, displacing some Streptococcus with the right Bacteroides might put things right again. Transitioning from male to female? Maybe a testosterone-degrading microbe could facilitate the process.
It’s a future of unprecedented agency, but it’s also a future full of hairy questions. Maybe we’ll find out that a bunch of people who want to transition male-to-female already have testosterone-degrading bacteria in their guts. “Doping” with synthetic steroids will get you banned from most competitive sports, so what happens if it turns out that 90% of Olympic medalists have a microbe that lets their body make more efficient use of their natural steroids? Should a probiotic like that be banned the way steroids are? And should people whose microbes naturally provide them with that kind of advantage compete in a separate category, or is that unfair to them?

That future is a while away, though. In the meantime, if anyone has GigaChad’s contact info, get at me. Just wanna see something.
—🖖🏼💩
P.S.: big thanks to reader Sarah Browne for catching me in a goof; the earlier version of this piece cited antibiotic use as surprisingly effective in treating endometriosis…turns out the study I was drawing on actually referred to endometritis, which is a very normal thing for antibiotics to be effective in treating.
